
Transport protein is a protein that can bind and carry distinctive molecules or ions from one organ to another organ. Examples are easy to transport protein myoglobin to store and distribute oxygen to the muscles, consider Figure 14:28.
Figure 14:28. Myoglobin, which distributes oxygen to the muscles
Hemoglobin is also a transport protein found in red blood cells. Hemoglobin can bind oxygen when the blood through the lungs. Oxygen was taken and released on peripheral tissues that can be used to oxidize nutrients (food) into energy. In blood plasma there is a lipoprotein that serves to transport lipids from the liver to the organ. Other transport proteins present in the cell membrane serves to bring some molecules such as glucose, amino acids and other nutrients through the membrane into the cell.
Both passive and active transport mediated by the help of a transmembrane protein that acts as a transporter. shows two major classes of transport proteins: carrier proteins and protein channels. For the most part, the carrier protein while the protein mediates the active transport channels mediate passive transport. Protein carrier makes a hole in the lipid bilayer to undergo conformational changes on the binding of molecules. Proteins form pores hydrophilic channel across the lipid bilayer. When open, these pores allow certain molecules to pass through. There is one other class of transport proteins called ionophores. It is a small, hydrophobic protein that increases the permeability of the double layer for a particular ion.
CARRIER PROTEINS move large molecules into or out of the cell down their concentration gradient. Different carrier proteins facilitated the diffusion of different molecules.
PROTEIN CHANNELS form pores in the membrane for charged particles to diffuse through (down their concentration gradient). Different protein channels facilitate the diffusion of different charged particles.

☞ ACTIVE TRANSPORT moves substances AGAINST a concentration gradient
Active transport uses ATP energy to move molecules and ions across plasma membranes, against a concentration gradient.
Carrier proteins:
- A molecule attaches to the carrier protein
- The protein changes shape
- This moves the molecule across the membrane
- ATP energy is used to move the solute against its concentration gradient
Co-transporters are a type of carrier protein:
- They bind two molecules at a time
- The concentration gradient of one of the molecules is used to move the other molecule against its own concentration gradient, essentially sucking it into the black hole that is the other side of the membrane
LIPID
Lipid refers to the group of aliphatic hydrocarbons nonpolar and hydrophobic. Because nonpolar, lipid insoluble in polar solvents such as water, but soluble in nonpolar solvents, such as alcohol, ether or chloroform. The most important biological functions of these lipids to store energy, as structural components of cell membranes and as signaling molecules.
Lipids are organic compounds derived from the dehydrogenation of hydrocarbons endotermal series. Lipids are amfifilik, meaning that lipids are able to form structures such as vesicles, liposomes, or other membranes in a wet environment. Biological lipids wholly or partly derived from two types subsatuan or "building blocks" of biochemistry: ketoasil group and isoprene groups. [4] Using this approach, lipids may be divided into eight categories: [5] fatty acyl, gliserolipid, gliserofosfolipid, sfingolipid , sakarolipid, and polyketides (derived from condensation subsatuan ketoasil), and sterol lipids and lipid prenol (derived from condensation of isoprene subsatuan).
Although the term lipid is sometimes used as a synonym of fat. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids, as well as sterol-containing metabolites such as cholesterol. [6] Although humans and mammals have the metabolism to break down and form a lipid, some lipids can not be generated in this way and must be obtained through food.
Examples of biologically important fatty acids are the eicosanoids, derived primarily from arachidonic acid and eicosapentaenoic acid, that include prostaglandins, leukotrienes, and thromboxanes. Docosahexaenoic acid is also important in biological systems, particularly with respect to sight.[13][14] Other major lipid classes in the fatty acid category are the fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as wax esters, fatty acid thioester coenzyme A derivatives, fatty acid thioester ACP derivatives and fatty acid carnitines. The fatty amides include N-acyl ethanolamines, such as the cannabinoid neurotransmitter anandamide.[15]
Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols found in plant membranes[18] and seminolipid from mammalian sperm cells.[19]
.[21] Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders.[22] Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.[23]

Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of or, themselves, membrane-derived second messengers.[24] Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.[25]
The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines),[28] whereas insects contain mainly ceramide phosphoethanolamines[29] and fungi have phytoceramide phosphoinositols and mannose-containing headgroups.[30] The glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
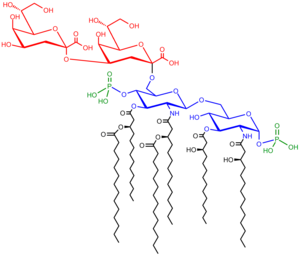
Saccharolipids describe compounds in which fatty acids are linked
directly to a sugar backbone, forming structures that are compatible
with membrane bilayers. In the saccharolipids, a monosaccharide
substitutes for the glycerol backbone present in glycerolipids and
glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides
of glucosamine, which are derivatized with as many as seven fatty-acyl
chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid
A, a hexa-acylated disaccharide of glucosamine that is glycosylated
with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.[41]
CARRIER PROTEINS move large molecules into or out of the cell down their concentration gradient. Different carrier proteins facilitated the diffusion of different molecules.
- First, a large molecule attaches to a carrier protein in the membrane.
- Then, the protein changes shape.
- This releases the molecule on the opposite side of the membrane.
PROTEIN CHANNELS form pores in the membrane for charged particles to diffuse through (down their concentration gradient). Different protein channels facilitate the diffusion of different charged particles.

☞ ACTIVE TRANSPORT moves substances AGAINST a concentration gradient
Active transport uses ATP energy to move molecules and ions across plasma membranes, against a concentration gradient.
Carrier proteins:
- A molecule attaches to the carrier protein
- The protein changes shape
- This moves the molecule across the membrane
- ATP energy is used to move the solute against its concentration gradient
Co-transporters are a type of carrier protein:
- They bind two molecules at a time
- The concentration gradient of one of the molecules is used to move the other molecule against its own concentration gradient, essentially sucking it into the black hole that is the other side of the membrane
LIPID
Lipid refers to the group of aliphatic hydrocarbons nonpolar and hydrophobic. Because nonpolar, lipid insoluble in polar solvents such as water, but soluble in nonpolar solvents, such as alcohol, ether or chloroform. The most important biological functions of these lipids to store energy, as structural components of cell membranes and as signaling molecules.
Lipids are organic compounds derived from the dehydrogenation of hydrocarbons endotermal series. Lipids are amfifilik, meaning that lipids are able to form structures such as vesicles, liposomes, or other membranes in a wet environment. Biological lipids wholly or partly derived from two types subsatuan or "building blocks" of biochemistry: ketoasil group and isoprene groups. [4] Using this approach, lipids may be divided into eight categories: [5] fatty acyl, gliserolipid, gliserofosfolipid, sfingolipid , sakarolipid, and polyketides (derived from condensation subsatuan ketoasil), and sterol lipids and lipid prenol (derived from condensation of isoprene subsatuan).
Although the term lipid is sometimes used as a synonym of fat. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids, as well as sterol-containing metabolites such as cholesterol. [6] Although humans and mammals have the metabolism to break down and form a lipid, some lipids can not be generated in this way and must be obtained through food.
Fatty acids
Fatty acids, or fatty acid residues when they form part of a lipid, are a diverse group of molecules synthesized by chain-elongation of an acetyl-CoA primer with malonyl-CoA or methylmalonyl-CoA groups in a process called fatty acid synthesis.[7][8] They are made of a hydrocarbon chain that terminates with a carboxylic acid group; this arrangement confers the molecule with a polar, hydrophilic end, and a nonpolar, hydrophobic end that is insoluble in water. The fatty acid structure is one of the most fundamental categories of biological lipids, and is commonly used as a building-block of more structurally complex lipids.[9] The carbon chain, typically between four and 24 carbons long,[10] may be saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen, and sulfur. Where a double bond exists, there is the possibility of either a cis or a trans geometric isomerism, which significantly affects the molecule's molecular configuration. Cis-double bonds cause the fatty acid chain to bend, an effect that is more pronounced the more double bonds there are in a chain. This in turn plays an important role in the structure and function of cell membranes.[11] Most naturally occurring fatty acids are of the cis configuration, although the trans form does exist in some natural and partially hydrogenated fats and oils.[12]Examples of biologically important fatty acids are the eicosanoids, derived primarily from arachidonic acid and eicosapentaenoic acid, that include prostaglandins, leukotrienes, and thromboxanes. Docosahexaenoic acid is also important in biological systems, particularly with respect to sight.[13][14] Other major lipid classes in the fatty acid category are the fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as wax esters, fatty acid thioester coenzyme A derivatives, fatty acid thioester ACP derivatives and fatty acid carnitines. The fatty amides include N-acyl ethanolamines, such as the cannabinoid neurotransmitter anandamide.[15]
Glycerolipids
Glycerolipids are composed mainly of mono-, di-, and tri-substituted glycerols,[16] the most well-known being the fatty acid triesters of glycerol, called triglycerides. The word triacylglycerol is sometimes used synonymously with triglyceride, however this is misleading with respect to these compounds as they contain no hydroxyl group. In these compounds, the three hydroxyl groups of glycerol are each esterified, typically by different fatty acids. Because they function as an energy store, these lipids comprise the bulk of storage fat in animal tissues. The hydrolysis of the ester bonds of triglycerides and the release of glycerol and fatty acids from adipose tissue are the initial steps in metabolising fat.[17]Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols found in plant membranes[18] and seminolipid from mammalian sperm cells.[19]
Glycerophospholipids
Glycerophospholipids, usually referred to as phospholipids, are ubiquitous in nature and are key components of the lipid bilayer of cells,[20] as well as being involved in metabolism and cell signaling.[21] Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders.[22] Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.[23]

Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of or, themselves, membrane-derived second messengers.[24] Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.[25]
Sphingolipids
Sphingolipids are a complicated family of compounds[26] that share a common structural feature, a sphingoid base backbone that is synthesized de novo from the amino acid serine and a long-chain fatty acyl CoA, then converted into ceramides, phosphosphingolipids, glycosphingolipids and other compounds. The major sphingoid base of mammals is commonly referred to as sphingosine. Ceramides (N-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fatty acid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 16 to 26 carbon atoms.[27]Sterol lipids
Sterol lipids, such as cholesterol and its derivatives, are an important component of membrane lipids,[31] along with the glycerophospholipids and sphingomyelins. The steroids, all derived from the same fused four-ring core structure, have different biological roles as hormones and signaling molecules. The eighteen-carbon (C18) steroids include the estrogen family whereas the C19 steroids comprise the androgens such as testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids.[32] The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure.[33] Other examples of sterols are the bile acids and their conjugates,[34] which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The plant equivalents are the phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter compound is also used as a biomarker for algal growth.[35] The predominant sterol in fungal cell membranes is ergosterol.[36]Prenol lipids
Prenol lipids are synthesized from the five-carbon-unit precursors isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via the mevalonic acid (MVA) pathway.[37] The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors of vitamin A.[38] Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin.[39] Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced.[40]Saccharolipids

Structure of the saccharolipid Kdo2-Lipid A.[41] Glucosamine residues in blue, Kdo residues in red, acyl chains in black and phosphate groups in green.




My problem
BalasHapus1.One way to prevent protein deficiency disease is by injecting the protein. The process of what happens to the protein injection?
The idea is injecting this protein can overcome the problem of malnutrition in Indonesia.
2.I want to ask about the problem of obesity.
obesity is excess body weight as a result of excessive accumulation of body fat.
Causes of Obesity
Scientifically, obesity is caused by consuming more calories than are required by the body.
The way the treatment is
1. With restricted caloric intake and increased physical activity or also called diet.Diet a regular and long-term
2. By way of liposuction. Liposuction has a high risk of death if done excessively. The ill effects of liposuction can range from blackened or wrinkled skin until bleeding and blood clots can be deadly.
Here I want to ask is where other treatments are best for obese people? Or there are other ways the most good?